SUVmax as Predictor of Metastatic Disease on 18F-FDG PET-CT in Hepatocellular Carcinoma
By Wardah Ashfaq1, Khurram Rehman2, Abubaker Shahid3, Muhammad Numair Younis1Affiliations
doi: 10.29271/jcpsp.2024.04.394ABSTRACT
Objective: To ascertain the utility of maximum standardised uptake value (SUVmax) in 18F-FDG PET-CT in predicting metastatic disease burden in hepatocellular carcinoma (HCC) patients.
Study Design: Descriptive study.
Place and Duration of the Study: Department of Nuclear Medicine and PET-CT Imaging, Institute of Nuclear Medicine and Oncology (INMOL), Lahore, Pakistan, from April to October 2022.
Methodology: 18F-FDG PET-CT data of 87 patients were analysed prospectively. Patients were considered regardless of resection status. The SUVmax measurements were performed, and their association with metastases was determined. Molecular docking studies were conducted to determine a mechanism behind the higher SUVmax at the metastatic sites.
Results: A higher number of patients (49) was found to have metastasis (1 to 5 in numbers) and demonstrated higher SUVmax, especially in cases of pre-surgery and post-transplant state. A positive correlation existed between SUVmax of pre-surgery (r = 0.419, p = 0.001) and post-transplant patients (r = 0.779, p = 0.001). Molecular docking studies revealed a strong binding affinity (-5.18± 0.25 kcal/mol) between the hexokinase (HK-II) and 18F-FDG.
Conclusion: SUVmax positively correlated with metastatic tumour burden. The strong binding affinity between the HK-II and 18F-FDG may be the reason. 18F-FDG PET-CT appeared beneficial in providing prognostic information for HCC in a selected group.
Key Words: Hepatocellular carcinoma, 18F-FDG, Positron emission tomography, Maximum standardised uptake value, SUVmax, HK-II binding, PET-CT, Metastases.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fourth most frequent aetiology of cancer-associated fatalities and ranked as the sixth most prevalent malignancy in the world.1 In Pakistan, statistics from a recognised hospital-based data registry show that HCC incidence has gradually increased, accounting for 10.7% of all malignancies.2 Only 10% of people with risk factors pre-disposing to HCC have access to routine screenings, and most patients are detected relatively late.3 HCC patients usually have a poor prognosis, and because of the many variables, including the tumour stage, alpha-fetoprotein (AFP), portal vein thrombosis, Child-Pugh score, and an increased likelihood of recurrence, estimating life expectancy is challenging.4
Detecting metastases and actual tumour burden in patients of HCC in early stages is crucial for effective treatment or, at minimum, improving quality of life. The diagnostic parameters in some serological and imaging techniques have been suggested that can estimate tumour burden and show association with the prognosis in HCC patients.5
Contrast-enhanced computed tomography (CECT) or magnetic resonance imaging (MRI) are commonly used in HCC patients. Positron emission tomography (PET) is a hybrid imaging modality that uses positron-emitting markers labelled with 2-deoxy-2-[18F] fluoro-D-glucose (18F-FDG), a glucose analogue. The dividing and growing cells experience a rise in 18F-FDG uptake because of the rapid glucose metabolism.6 18F-FDG PET, in addition to being a whole-body technique, when fused with CT, has high sensitivity in diagnosing and staging malignancies and is utilised as a response assessment tool in managing patients. However, the diagnostic performance of 18F-FDG PET-CT is restricted in HCC because of an inconsistent pattern of 18F-FDG uptake.
18F-FDG accumulation in HCC is heterogeneous depending on the histopathological differentiation of the tumour, and thus 18F-FDG PET-CT has demonstrated a sensitivity of 50-70% for identification of primary HCC.7 Poorly differentiated HCC shows increased 18F-FDG uptake as compared to well-differentiated variety, and it aids in the identification of the distant metastases as poorly differentiated type is more prone to metastasise.8 The degree of differentiation and degree of FDG uptake by the primary tumour are closely related. 18F-FDG PET-CT imaging has been associated with a degree of differentiation of malignant cells and seemed to provide the risk of tumour relapse and longevity in the HCC patients who underwent surgical resection.5 The 18F-FDG PET-CT is ranked higher as compared to other techniques in identifying occult metastases and is more accurate as compared to CT in differentiating intrahepatic malignancies (88.9% vs. 81.5%).6 This study analysed the maximum standardised uptake value (SUVmax) on 18F-FDG PET-CT among HCC patients and any variation in these values as an indicator of metastatic disease. Also, the possible molecular mechanism was determined behind 18F-FDG accumulation in HCC through computerised molecular docking studies. Keeping in view the metabolic analytical ability of FDG PET-CT, the aim of this study was to ascertain the utility of maximum standardised uptake value (SUVmax) in 18F-FDG PET-CT in predicting metastatic disease HCC patients and identify it as a biomarker for metastatic HCC.
METHODOLOGY
A total of 90 patients underwent 18F-FDG PET-CT for HCC at INMOL Hospital during the study period of six months i.e. April to October 2022. The inclusion criteria were adult patients who had confirmed diagnosis of HCC and those who had given consent were included in the study. Patients with dual malignancies were excluded from this study. After applying the inclusion and exclusion criteria, eighty-seven consecutive patients with HCC were analysed.
Each patient had undergone a conventional diagnostic work-up, CECT and was then referred for 18F-FDG PET-CT. Fifty-six patients were referred for 18F-FDG PET-CT before starting their treatment; fourteen patients had liver transplants, and seventeen underwent TACE out of these eighty-seven patients. All enrolled patients provided prior informed consent, and the institution approved the study protocol.
18F-FDG PET-CT scans were acquired on the Discovery XT GE health-care machine according to the EANM 2.0 guidelines.9 Patients received 2.7 MBq/kg of radiotracer intravenously with a fasting serum glucose reading <200mg/dl. Both sets of PET and CT images were viewed in isolation and fusion modes using the Fusion software ADW 4.4 version (GE Healthcare), as shown in Figure 1, which also indicates a focal hypermetabolic lesion in the spleen consistent with metastatic HCC. The SUVmax values were evaluated by drawing rectangular regions of interest over the pathological sites showing high FDG uptake in liver and metastatic sites.
Molecular docking is a renowned method for determining small molecules' optimal orientation and receptor binding affinity.10,11 HCC proteins and the 18F-FDG were molecularly docked using free, open-source software, including AutoDockVina from PyRx Virtual Screening and BIOVIA Discovery Studio 2017. (San Diego, CA). Calculations of energy (kcal/mol) and binding affinity were performed using the AutoDockVina screening software. The BIOVIA Discovery Studio 2017 was used for virtual investigation. All water molecules and cofactors were omitted. A ligand library was created after the three-dimensional (3D) structures were collected from the protein data bank (PDB). Eventually, docking investigations were conducted once all structures were optimised using energy minimisation.
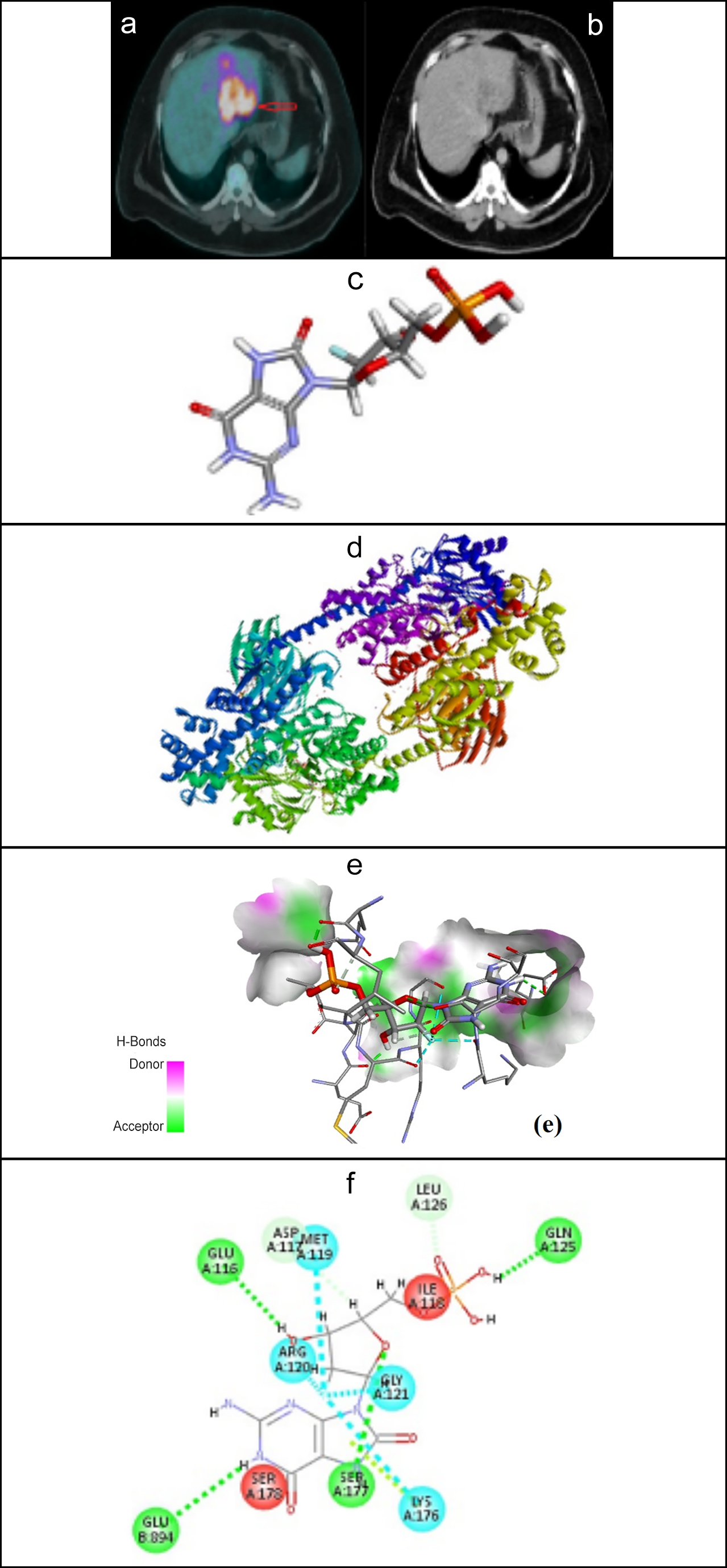 Figure 1: (a) Fused axial PET-CT image of HCC demonstrating high uptake of 18F-FDG in HCC (b) Axial CT image at the same level, (c) Structure of 18F-FDG (d) Structure of human hexokinase-II (e) H-Bonds donor and acceptor regions and (f) key interactions between HK-II and 18F-FDG.
Figure 1: (a) Fused axial PET-CT image of HCC demonstrating high uptake of 18F-FDG in HCC (b) Axial CT image at the same level, (c) Structure of 18F-FDG (d) Structure of human hexokinase-II (e) H-Bonds donor and acceptor regions and (f) key interactions between HK-II and 18F-FDG.
Table I: Analysis of number of metastasis and SUVmax value.
|
Patient Groups |
N |
Mean Rank |
Sum of Ranks |
Asymp. Sig. (2-tailed) |
|
Patients SUV (without Metastases) |
38 |
37.25 |
1415.50 |
0.028 |
|
Patients SUV (with Metastases) |
49 |
49.23 |
2412.50 |
|
|
Total |
87 |
|
|
|
|
Pearson Correlation |
N |
Correlation |
Sig. (2-tailed) |
|
|
1. Patients SUV and Number of Metastases |
87 |
0.486 |
0.000 |
|
|
2. Pre-surgery SUV and Number of Pre-surgery Metastases |
56 |
0.419 |
0.001 |
|
|
3. Post-Transplant SUV and Number of Post-Transplant Metastases |
14 |
0.779 |
0.001 |
|
|
4. Post-TACE SUV and Number of Post-TACE Metastases |
17 |
0.297 |
0.247 |
|
|
The Mann-Whitney test shows patients with evidence of metastasis have higher mean rank correlated with higher SUVmax value and Pearson Correlation between the SUVmax and number of metastases. |
||||
Both parametric and non-parametric different statistical models were used for data analysis. Pearson correlation was found for the parametric data, which included the correlation of SUVmax to the total number of metastases in a patient. The p-values <0.05 were statically significant in all patients. The Mann-Whitney U test was conducted for the non-parametric data, which included the correlation of SUVmax with whether any evidence of metastatic disease is present in a patient. SPSS version 15.0 software was used. The calculations were performed for both confidence levels of 95% and 99%. The results showed that 73 patients were required for the 95% confidence level and 80 patients for the 99% confidence level analysis. Therefore, for this study, and considering possible dropouts the data were analysed for the maximum number of patients as possible, which was 87.
RESULTS
Of the 87 patients with an average age of 53±9.7 years, 72 (82.76%) were males and 15 (17.24 %) were females, demonstrating an SUVmax mean value of 3.48. Fifty-six were pre-surgery cases with a mean SUVmax value of 3.45, fourteen were post-transplant cases with a mean SUVmax value of 3.67, and seventeen were post-TACE patients with a mean SUVmax value of 3.05.
To confirm an association between SUVmax and the evidence of metastases, the patients were divided into two groups: patients without any evidence of metastases (38 patients) and patients with a confirmed metabolic disease (49 patients). Then, using the Mann-Whitney test, it was confirmed that the patients with evidence of metastasis have higher mean rank (Table I) correlated with higher SUVmax values, demonstrating a statistically significant (p=0.028) difference between the two groups.
Of forty-nine patients with metastases, the total number of metastases for each patient was also counted. This number ranged from 1 to 5, as no patient had more than 5 metastatic sites. Among eighty-seven patients, 38 patients (43.67%) had no metastases, 29 patients (33.33%) had 1 metastatic site only, 15 patients (17.24%) had 2 metastatic sites, 2 patients (2.29%) had 3 and 4 metastatic sites, whereas only 1 patient (1.14%) had 5 metastatic sites. No patient had more than 5 metastatic sites. The Pearson correlation indicated that the SUVmax positively correlated to the total number of metastases (r = 0.486, p <0.001). The Pearson correlation results indicated a moderately positive correlation present between the SUVmax value, suggesting that as the SUVmax increases, there is a good probability that there is an increased risk of metastases, as shown in Table I.
The SUVmax of pre-surgery, post-transplant, and post-TACE cases was also evaluated to find a correlation between the SUVmax and metastases evidence. This analysis would help to determine the usefulness of 18F-FDG PET-CT as a predictive or diagnostic parameter for HCC metastases shown in Table I. First, the Pearson correlation analysis was conducted for the pre-surgery patients. The results were statistically significant and indicated a moderately positive correlation between SUVmax and the number of metastases (r = 0.419, p = 0.001), suggesting that as the SUVmax increases, there is a good probability that there is an increased risk of metastases. More interestingly, when the Pearson correlation analysis was repeated for the post-transplant patients, the results showed a statistically significant strong positive correlation between SUVmax and the number of metastases (r = 0.779, p = 0.001). This strong positive correlation suggests that SUVmax value as a diagnostic or predictive method for metastasis may be more suitable in the case of post-transplant patients. However, in the case of post-TACE patients, the Pearson correlation analysis showed a weak positive correlation between SUVmax and number of metastasis, nevertheless, the results were not statistically significant (r = 0.297, p = 0.247). This suggests that SUVmax might not be an ideal predictive tool for post-TACE patients.
Evaluation of the affinity between HK-II and 18F-FDG was performed to identify the cause for the binding affinity between the two molecules to understand the underlying reason for the accumulation of 18F-FDG in HCC tumour cells. The average molecular binding energy between HK-II and the 18F-FDG was found to be -5.18± 0.25 kcal/mol. The studies were also conducted to propose the binding site, bond length, and interactions between HK-II and 18F-FDG (shown in Figure 1). It was found that different interactions, such as conventional hydrogen bonds and halogen interactions, exist between HK-II and 18F-FDG, as shown in Table II. 18F-FDG could interact with HK-II through SER-177, GLU-894, GLU-116, LEU-126, ASP-117, MET-119, GLY-129, and LYS-176. In general rule, the shorter the bond distance the stronger will be the bond interaction between the two molecules. ASP-117 and GLU-116 showed the strongest hydrogen bonding interaction with the 18F-FDG. The details of the interaction positioning and the bond length present between each interaction are given in Table II.
Table II: Interaction details between the HK-II and 18F-FDG, their category and the bond distance.
|
Name |
Category |
Distance Ao |
|
A:SER177:OG - FDG:O20 |
Hydrogen Bond |
3.01681 |
|
FDG:H25 - B:GLU894:OE1 |
Hydrogen Bond |
2.78411 |
|
FDG:H32 - A:GLU116:O |
Hydrogen Bond |
1.89548 |
|
A:LEU126:CA - FDG:O23 |
Hydrogen Bond |
2.25461 |
|
FDG:H33 - A:ASP117:O |
Hydrogen Bond |
1.91025 |
|
A:MET119:O - FDG:F16 |
Halogen |
2.75542 |
|
A:GLY121:O - FDG:F16 |
Halogen |
3.55288 |
|
A:LYS176:O - FDG:F16 |
Halogen |
2.68532 |
DISCUSSION
As an indicator of metabolic activity, 18F-FDG PET-CT has the prime advantage in the work-up and follow-up of malignant diseases by providing a map of metabolic activity in the tumour and distant locations of metastasis. Due to the increased expression of glucose transporters (GLUT-2) and hexokinase-II (HK-II) activity, most cancers rely on glucose as their prime energy source.12 Many studies have reported moderate 18F-FDG uptake and strong expression of GLUT-2 and HK-II in the case of hepatocellular carcinoma.13,14 Hexokinase effectively phosphorylates 18F-FDG to 18F-FDG -6 phosphate by binding to the mitochondrial membrane and phosphorylation prevents 18F-FDG from exiting the cell leading to accumulation there instead.11,15 This entrapment in tumour cells provides a map of metabolic activity when imaged and enables use of docking studies.
Normal hepatocytes have a high concentration of glucose-6-phosphatase and a low concentration of hexokinase, but HCC cells have the opposite ratio. 18F-FDG can concentrate in HCC but not in healthy cells due to this imbalance.16 According to reports, the sensitivity of 18F-FDG PET-CT for HCC detection varies from 50 to 70% depending on the amount of this enzyme present in HCC cells.17 CT and MRI perform better in HCC detection, yet they cannot distinguish well-differentiated from poorly-differentiated HCC. The variability of 18F-FDG uptake has been linked to the differentiation and mitotic activity of HCC, and as biopsy is not the gold standard in cases of HCC, 18F-FDG PET-CT may be utilised as a non-invasive tool for assessing tumour features and behaviour.18,19
The present study evaluated SUVmax values' usefulness in 18F-FDG PET-CT predicting the prognosis of metastatic disease in HCC. Figure 2 shows an image of a patient with HCC and metastases at various sites. A higher SUVmax showed increased evidence of metastatic disease in pre-surgery and post-transplant patients. However, only a moderately positive correlation was found in post-TACE patients, considering that the TACE is offered only to patients without any biochemical and image-based evidence of metastasis. Post-transplant patients showed a strong positive correlation between SUVmax and metastases, favouring its use as a diagnostic tool to determine the potential of HCCs recurrence or metastasis. These results also correlate with other studies that found a relationship between SUVmax and a higher incidence of metastases for diagnosis.4
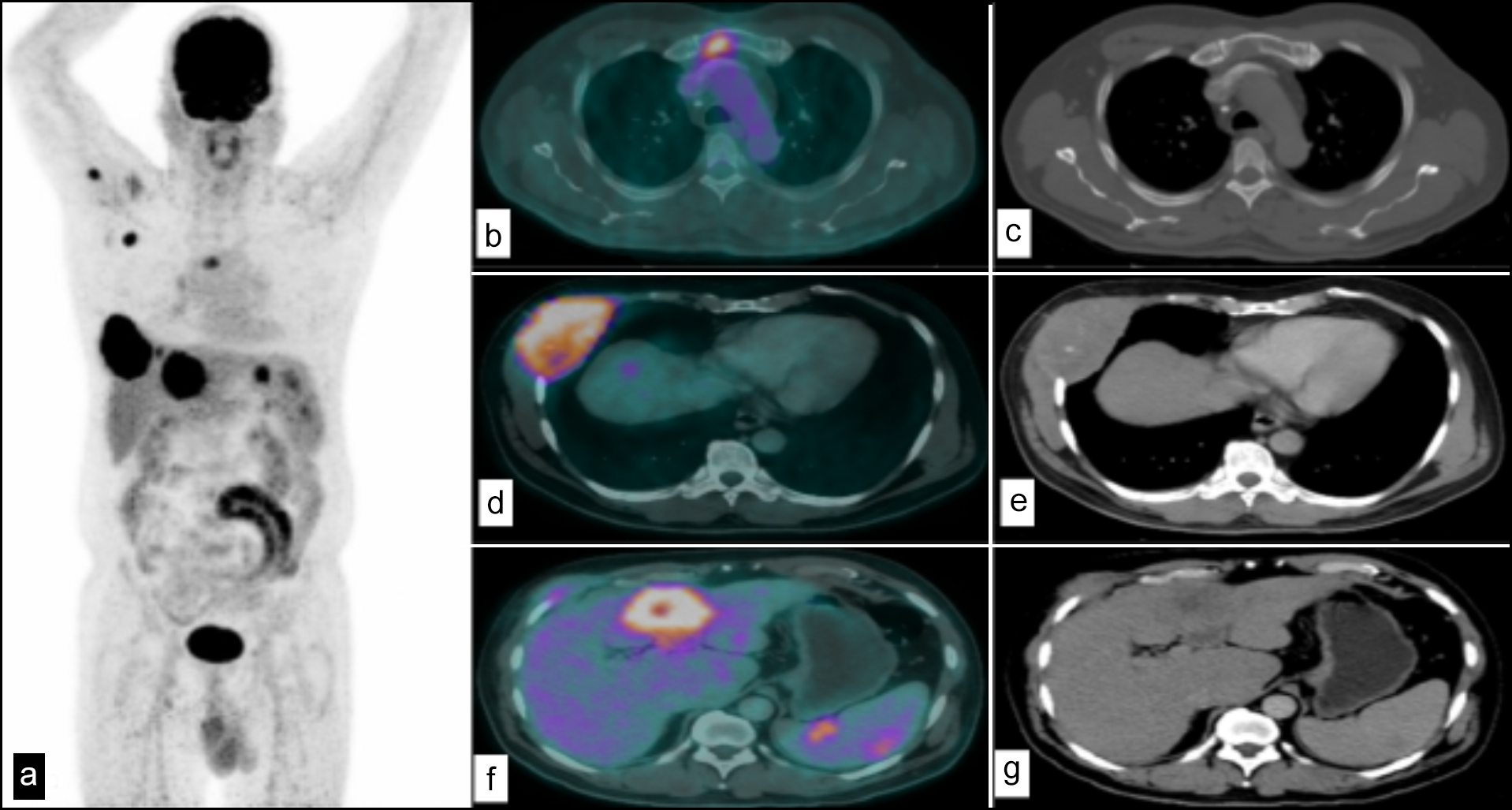 Figure 2: (a) MIP image of a patient with HCC and metastases at various sites. (b) Fused axial PET-CT image with metastatic hypermetabolic focus in sternum. (c) CT image showing a lytic focus in sternum. (d) Fused axial PET-CT image showing hypermetabolic soft tissue density mass involving right sided ribs and intercostal muscles. (e) CT image showing soft tissue density mass involving right sided ribs and intercostal muscles. (f) Primary hypermetabolic HCC lesion in liver. (g) CT image showing irregular shaped hypodense area in liver-primary HCC.
Figure 2: (a) MIP image of a patient with HCC and metastases at various sites. (b) Fused axial PET-CT image with metastatic hypermetabolic focus in sternum. (c) CT image showing a lytic focus in sternum. (d) Fused axial PET-CT image showing hypermetabolic soft tissue density mass involving right sided ribs and intercostal muscles. (e) CT image showing soft tissue density mass involving right sided ribs and intercostal muscles. (f) Primary hypermetabolic HCC lesion in liver. (g) CT image showing irregular shaped hypodense area in liver-primary HCC.
The SUVmax has been shown as one of the critical prognostic indicators.20 Thus, an analysis of 18F-FDG binding affinity with enzymes responsible for its metabolism in the tumour cells was done. The metabolic activity of a tissue can be ascertained from the quantity of 18F-FDG collected in the tissue over a specific time. 18F-FDG accumulation strongly relies on GLUTs and the rate-limiting glycolytic HK-II in most malignancies.11,21 For effective glucose consumption, it is critical to have enough HK-II protein, particularly in the mitochondrial bond form in cancer cells. In HCC, for example, mitochondrial-bound hexokinase can represent approximately 70% of the total cellular hexokinase, whereas the amount on the mitochondria of normal liver cells is negligible.22 Therefore, this study conducted molecular docking-based virtual screening between 18F-FDG and HK-II to better understand the possible mechanism behind the 18F-FDG uptake in the tumour cells. The results reported strong binding energy between the molecules of 18F-FDG and HK-II.
Upon binding of a molecule to a target, the total energy of the complex is reduced due to the release of binding energy. Also, any modification of the ligand brought on by its minimum energy in relation to its conformation when bound to the macromolecule is made up for by the release of energy equal to its binding energy. Thus, a ligand has a stronger propensity to connect with a given macromolecule, the more energy is generated during the binding process.23,24 The negative binding energy suggests that the 18F-FDG was bound to HK-II spontaneously without needing any energy. Furthermore, the hydrogen interactions also existed between the 18F-FDG and HK-II, which indicates why 18F-FDG is accustomed to being bound with HK-II and entrapped in the tumour cells, which eventually results in higher SUVmax values. The docking studies in the study population were not performed to show a difference in SUVmax uptake values among groups of patients with well-differentiated and poorly differentiated carcinoma, as histopathology of all patients was unavailable. However, docking studies have been performed by Song et al. to validate that poorly differentiated HCC hexokinase expression increases, leading to high 18F-FDG uptake and retention in cells, explaining the possible reason behind the higher SUVmax in these patients.25,26
Further studies involving patients from multiple centres and longer follow-up is suggested to validate these findings. The study population was heterogeneous, comprising pre-surgery, post-transplant, and post-TACE patients due to time constraints. Docking studies could not show a difference in SUVmax in patients with well-differentiated and poorly differentiated HCC because histopathology of all patients was not available.
CONCLUSION
A positive correlation was demonstrated between the SUVmax and the HCC metastases and suggested that 18F-FDG PET-CT can be presented as a predictive marker to diagnose the metastases of HCCs. The mechanism behind the higher SUVmax in tumour cells is likely to be a higher affinity for HHC- II binding in HCC metastases sites.
ETHICAL APPROVAL:
The present study was approved by the Ethics Committee of the Institute of Nuclear Medicine and Oncology, Lahore, Pakistan.
PATIENTS’ CONSENT:
All enrolled patients provided prior informed consent.
COMPETING INTEREST:
The authors declared no conflict of interest.
AUTHORS’ CONTRIBUTION:
MNY, WA: Conception, design of the work, and drafting the manuscript.
WA, KR: Data collection, data analysis, and interpretation.
MNY: Critical revision of the manuscript.
AS, MNY: Study supervision, funding, and materials, etc.
All authors approved the final version of the manuscript to be published.
REFERENCES
- Samant H, Amiri HS, Zibari GB. Addressing the worldwide hepatocellular carcinoma: Epidemiology, prevention and management. J Gastrointest Oncol 2021; 12(2):361-73. doi: 10.21037/jgo.2020.02.08.
- Hafeez Bhatti AB, Dar FS, Waheed A, Shafique K, Sultan F, Shah NH. Hepatocellular carcinoma in Pakistan: National trends and global perspective. Gastroenterol Res Pract 2016; 2016:5942306. doi: 10.1155/2016/5942306.
- Tahir M. Hepatocellular carcinoma: Hope and challenges. Liaquat N J Can Care 2021; 3(1):1-2. doi: 10.37184/lnjcc. 2789-0112.3.8.
- Trojan J, Schroeder O, Raedle J, Baum RP, Herrmann G, Jacobi V, et al. Fluorine-18 FDG positron emission tomography for imaging of hepatocellular carcinoma. Am J Gastroenterol 1999; 94(11):3314-9. doi: 10.1111/j.1572- 0241.1999.01544.x.
- Xia H, Chen J, Gao H, Kong SN, Deivasigamani A, Shi M, et al. Hypoxia-induced modulation of glucose transporter expression impacts 18F-fluorodeoxyglucose PET-CT imag-ing in hepatocellular carcinoma. Eur J Nucl Med Mol Imaging 2020; 47(4):787-97. doi: 10.1007/s00259-019-04 638-4.
- Zhu A, Lee D, Shim H. Metabolic positron emission tomography imaging in cancer detection and therapy response. Semin Oncol 2011; 38(1):55-69. doi:10.1053/j.seminoncol. 2010.11.012.
- Asman Y, Evenson RA, Even-Sapir E, Shibolet O. [18F]Fludeoxyglucose positron emission tomography and computed tomography as a prognostic tool before liver transplantation, resection, and loco-ablative therapies for hepatocellular carcinoma. Liver Transpl 2015; 21(5): 572-80. doi:10.1002/lt.24083.
- Moon CM, Bang S, Chung JB, Park SW, Song SY, Yun M, et al. Usefulness of 18F-fluorodeoxyglucose positron emission tomography in differential diagnosis and staging of cholangiocarcinomas. J Gastroenterol Hepatol 2008; 23(5): 759-65. doi:10.1111/j.1440-1746.2007.05173.x.
- Boellaard R, Delgado-Bolton R, Oyen WJG, Giammarile F, Tatsch K, Eschner W, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging 2015; 42(2):328-54. doi: 10.1007/s00259- 014-2961-x.
- Khan A, Mohammad T, Shamsi A, Hussain A, Alajmi MF, Husain SA, et al. Identification of plant-based hexokinase 2 inhibitors: Combined molecular docking and dynamics simulation studies. J Biomol Struct 2022; 40(20):10319-31. doi:10.1080/07391102.2021.1942217.
- Kilicoglu O, Sepay N, Ozgenc E, Gundogdu E, Kara U, Alomairy S, et al. Evaluation of F-18 FDG radiopharmaceuticals through molecular docking and radiation effects. Appl Radiat Isot 2023; 191:110553. doi: 10.1016/j.apradiso. 2022.110553.
- Yang H, Zhong JT, Zhou SH, Han HM. Roles of GLUT-1 and HK-II expression in the biological behavior of head and neck cancer. Oncotarget 2019; 10(32):3066-83.
doi:10.18632/oncotarget.24684. - Tulin PE, Dolgushin MB, Odzharova AA, Mikhaylov AI, Medvedeva BM, Shiryaev SV, et al. Perfusion CT and PET with 18F-FDG and 18F-FCh in the complex diagnosis of hepatocellular carcinoma. Eur J Hybrid Imaging 2017; 1(1):13. doi:10.1186/s41824-017-0018-7.
- Gnocchi D, Sabbà C, Massimi M, Mazzocca A. Metabolism as a new avenue for hepatocellular carcinoma therapy. Int J Mol Sci 2023; 24(4):3710. doi:10.3390/ijms24043710.
- Lee M, Ko H, Yun M. Cancer metabolism as a mechanism of treatment resistance and potential therapeutic target in hepatocellular carcinoma. Yonsei Med J 2018; 59(10): 1143-9. doi:10.3349/ymj.2018.59.10.1143.
- Sarikaya I, Schierz JH, Sarikaya A. Liver: Glucose metabolism and 18F-fluorodeoxyglucose PET findings in normal parenchy-ma and diseases. Am J Nucl Med Mol Imaging 2021; 11(4):233-49.
- Ozaki K, Harada K, Terayama N, Kosaka N, Kimura H, Gabata T. FDG-PET/CT imaging findings of hepatic tumors and tumor-like lesions based on molecular background. Jpn J Radiol 2020; 38(8):697-18. doi:10.1007/s11604-020-00961-1.
- Kornberg A, Friess H. 18F-fludeoxyglucose positron emission tomography for diagnosis of HCC: implications for therapeutic strategy in curative and non-curative approaches. Therap Adv Gastroenterol 2019; 12:1756284819836205. doi:10.1177/1756284819836205.
- Abuodeh Y, Naghavi AO, Ahmed KA, Venkat PS, Kim Y, Kis B, et al. Prognostic value of pre-treatment F-18-FDG PET-CT in patients with hepatocellular carcinoma undergoing radioembolization. World J Gastroenterol 2016; 22(47):10406-14. doi:10.3748/wjg.v22.i47.10406
- Li D, Wang Y, Liu W, Chen Q, Cai L, Xing X, et al. The correlation between 18F-FDG PET/CT imaging SUVmax of preoperative colon cancer primary lesions and clinicopathological factors. J Oncol 2021; 2021:4312296. doi:10.1155/2021/ 4312296.
- Temre MK, Kumar A, Singh SM. An appraisal of the current status of inhibition of glucose transporters as an emerging antineoplastic approach: Promising potential of new pan-GLUT inhibitors. Front Pharmacol 2022; 13:1035510. doi:10.3389/fphar.2022.1035510.
- Bustamante E, Morris HPa, Pedersen PL. Energy metabolism of tumor cells. Requirement for a form of hexokinase with a propensity for mitochondrial binding. J Biol Chem 1981; 256(16):8699-704.
- Dai Y, Wang Q, Zhang X, Jia S, Zheng H, Feng D, et al. Molecular docking and QSAR study on steroidal compounds as aromatase inhibitors. Eur J Med Chem 2010; 45(12): 5612-20. doi:10.1016/j.ejmech.2010.09.011.
- Zulfakar MH, Chan LM, Rehman K, Wai LK, Heard CM. Coenzyme Q10-loaded fish oil-based bigel System: Probing the delivery across porcine skin and possible interaction with fish oil fatty acids. AAPS Pharm Sci Tech 2018; 19(3): 1116-23. doi:10.1208/s12249-017-0923-x.
- Song C, Gu X, Li R. Expression of IRAK1 in hepatocellular carcinoma, its clinical significance, and docking characteristics with selected natural compounds. Curr Oncol 2022; 29(11):8904-16. doi:10.3390/curroncol29110700.
- Arshad K, Hanan SD, Younis MN, Badar R, Imran M, Numair N, et al. Detection of Primary Hepatocellular Carcinoma on 18F-Fluorodeoxyglucose Positron Emission Tomography-computed Tomography. Euroasian J Hepatogastroenterol 2023: 13(2):66-72. doi: 10.5005/jp-journals-10018-1409.